PitStop
|
Brochure
|
|
Surgical Technique
|
Cannulated Insertion
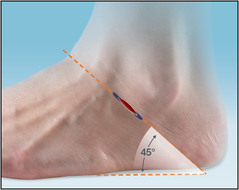
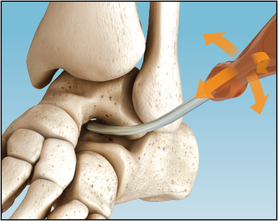
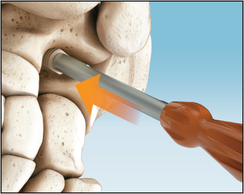
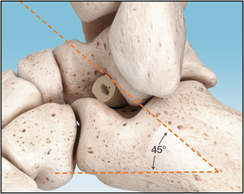
The Inserter is used to press fit the Implant in the correct position with a pushing motion. Do not screw Implant into place. X-ray markers at each end of the Implant help achieve the adequate depth with fluoroscopy visualization. The corresponding flat surfaces of the Implant and Inserter handle are aligned parallel to the lateral talar process, which is an approximate 45° angle to the fibula and the plantar aspect of the foot on the lateral view.
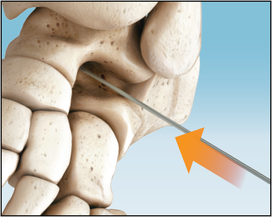
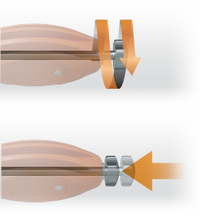
Unscrew the Internal Holder to release the Implant and push with finger pressure to remove the Holder from the Implant.
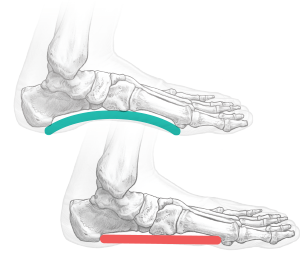
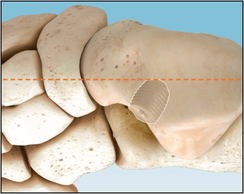
Hindfoot mobility is assessed to verify adequate correction. The wound should be closed according to surgeon preference.
The Inserter is used to press fit the Implant in the correct position with a pushing motion. Do not screw Implant into place. X-ray markers at each end of the Implant help achieve the adequate depth with fluoroscopy visualization. The corresponding flat surfaces of the Implant and Inserter handle are aligned parallel to the lateral talar process, which is an approximate 45° angle to the fibula and the plantar aspect of the foot on the lateral view.
Unscrew the Internal Holder to release the Implant and push with finger pressure to remove the Holder from the Implant.
Hindfoot mobility is assessed to verify adequate correction. The wound should be closed according to surgeon preference.
CAUTION: Federal law (USA) restricts this device to sale and use by, or on the order of, a physician.
All content contained herein is furnished for informational purposes only. In2Bones does not recommend a particular surgical product or procedure suitable for all patients. Each surgeon must evaluate the appropriateness of a device and corresponding techniques based on medical training, clinical judgment and surgical experience. The proper surgical technique and/or procedure are the responsibility of the medical professional. Indications, contraindications, warnings and precautions are listed in the implant package insert and should be reviewed carefully by the physician and operating room personnel prior to any proposed procedure. Availability of these products might vary from one given country or region to another as a result of specific local regulatory approval or clearance requirements for sale in such country or region.
All content contained herein is furnished for informational purposes only. In2Bones does not recommend a particular surgical product or procedure suitable for all patients. Each surgeon must evaluate the appropriateness of a device and corresponding techniques based on medical training, clinical judgment and surgical experience. The proper surgical technique and/or procedure are the responsibility of the medical professional. Indications, contraindications, warnings and precautions are listed in the implant package insert and should be reviewed carefully by the physician and operating room personnel prior to any proposed procedure. Availability of these products might vary from one given country or region to another as a result of specific local regulatory approval or clearance requirements for sale in such country or region.